american academy of pediatrics covid vaccine pregnancy
18 2021 - Roughly 26 million children ages 5-11 years have got a COVID-19 vaccine in the first two weeks of eligibility. 16 2021 - The timing of COVID testing depends on whether patients have symptoms and whether they are fully vaccinated.

Information And Resources For Covid 19 And Breastfeeding
There is no recommendation to postpone breastfeeding or discard breast milk after getting the vaccine.

. Factors such as mothers underlying health conditions and demographic variables also were considered. April 28 2022. One study also looked at vaccination status.
Patients who are pregnant may be at an increased risk of severe illness from COVID-19 and adverse events such as preterm birth. This includes promoting nurturing inclusive. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.
A new report on COVID-19 vaccines from the Kaiser Family Foundation emphasizes the challenges family physicians and other health care professionals are facing as they try to combat misinformation. Injection-site pain was reported more frequently among pregnant persons than among nonpregnant women whereas headache myalgia chills and fever were reported less frequently. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age.
However more longitudinal follow-up including follow-up of large numbers of. 29 the FDA authorized use of the Pfizer-BioNTech COVID-19 vaccine for children 5-11 years old. A total of 35691 v-safe participants 16 to 54 years of age identified as pregnant.
Pregnant people also are included in the CDCs clinical considerations for use of COVID-19 vaccines. The AAP calls for the on-time routine immunization of all children and adolescents according to its policy Recommended Immunization Schedules for Children and Adolescents Aged 18 Years or. The American Academy of Pediatrics AAP in collaboration with the Centers for Disease Control and Prevention CDC conducted 9 key informant interviews with emerging pediatric practices to identify innovative strategies to continue conducting developmental surveillance screening referral and follow-up during the COVID-19 pandemic.
Although patients who were pregnant or. Organizations including the Academy of Breastfeeding Medicine and the American Academy of Pediatrics agree that people who are breastfeeding can receive COVID-19 vaccines. Pregnant women only had slightly higher rates of injection-site pain.
COVID-19 vaccination is the best method to reduce maternal and fetal complications of COVID-19 infection among pregnant people said William Grobman MD MBA president of SMFM. Among 3958 participants enrolled in the v-safe pregnancy registry 827 had a. The AAFP applauds the actions by the FDA protect children from the devastating effects of.
Breastfeeding and COVID-19 vaccines. In todays COVID-19 Update representatives from the American College of Obstetricians and Gynecologists ACOG Linda Eckert MD and the American Academy of Pediatrics AAP Flor Muñoz-Rivas MD share COVID-19 vaccine guidance for pregnant and lactating individuals. Pregnant and nursing patients were excluded from the vaccine trials but patients did get pregnant during the trials and there have been no complications reported.
Adolescents age 12-17 years are only eligible for the Pfizer-BioNTech COVID-19 vaccine These recommendations also apply to pregnant and recently pregnant eg up to 6 weeks postpartum individuals who completed their initial COVID-19 vaccine or vaccine series prior to pregnancy. The American Academy of Pediatrics is encouraged that the US. Preliminary findings did not show obvious safety signals among pregnant persons who received mRNA Covid-19 vaccines.
The American College of Obstetricians and Gynecologists ACOG is following the situation closely. Maximize protection get all recommended vaccine doses and boosters as soon as possible. Additionally recent studies show that vaccination during pregnancy with a 2-dose mRNA COVID-19 vaccine series may help prevent COVID-19 hospitalizations in infants 6 months of age.
Learn more about the American Academy of Pediatrics including our mission leadership and commitment to the optimal health and well-being of all children. Moderna has released animal. Maternal-fetal medicine subspecialistsexperts in high-risk pregnancy strongly recommend that pregnant people get vaccinated.
Right now children and adolescents 5 to under 18 in the US. American Academy of Pediatrics Urges Quick and Transparent Steps Toward Review of COVID-19 Vaccine for Youngest Age Group of Children. Nucleic acids found naturally in all our cells instructs them to make protein.
AAP updates interim guidance on COVID testing face masks MIS-C Nov. Data from tens of thousands of reporting individuals have shown that the COVID-19 vaccine is both safe and effective when administered during pregnancy. Safety and Efficacy for the Newborn.
Ad Safety is CDCs top priority and vaccination is the safest way to help build protection. Moreover COVID-19 vaccines have no impact on fertility. The new reports analyzed maternal or perinatal outcomes in three similar time periods.
Start the day smarter Notable deaths in. Experts are learning more every day about the new coronavirus that causes COVID-19. Statement by Lee Savio Beers MD FAAP president of the American Academy of Pediatrics.
COVID-19 mRNA vaccines contain messenger RNA mRNA which is made up of nucleic acids. The American Academy of Pediatrics helped parents understand COVID with faster guidelines than the CDCs. The American Academy of Pediatrics strongly recommends immunizations as the safest and most cost-effective way of preventing disease disability and death.
Food and Drug Administration has authorized a COVID-19 vaccine for children ages 5 to 11 after reviewing scientific data validating its safety and effectiveness. ACOG recommends that pregnant and recently pregnant people up to 6 weeks postpartum receive a booster dose of COVID-19 vaccine following the completion of their initial COVID-19 vaccine or vaccine series. Data from 35692 pregnant women from the v-safe monitoring system found the most common side effects to be the same as non-pregnant women injection-site pain fatigue headache and myalgia according to the NEJM study.
Learn more at the AMA COVID-19 resource center. From the beginning the American Academy of Pediatrics has been guided by its mission to ensure the health and well-being of all children. This page will be updated as ACOG learns new information for pregnant and.
Heres what the AAP says on kid vaccines. Can only receive the COVID-19 mRNA vaccine from Pfizer BioNTech. The same data have been equally reassuring when it comes to infants born to vaccinated individuals.

Covid 19 Vaccines And Children State Strategies To Increase Access And Uptake Through Pediatric Providers The National Academy For State Health Policy
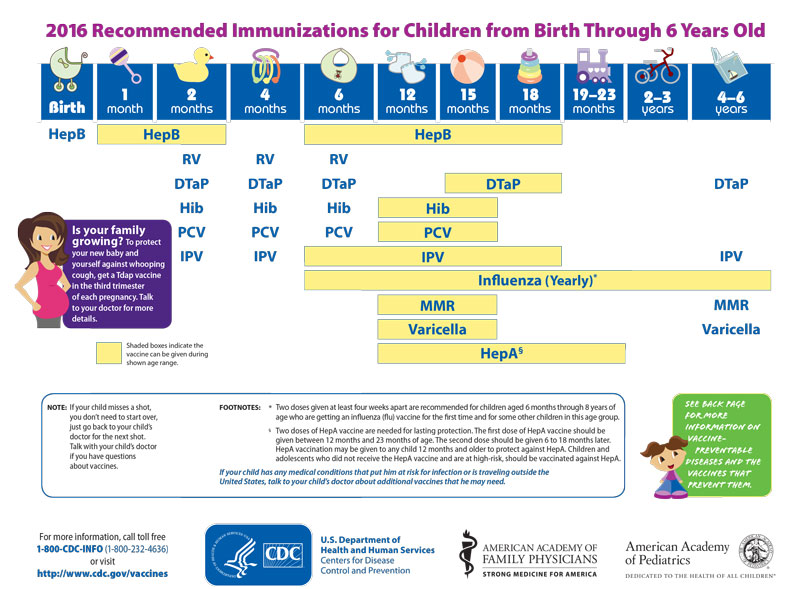
Immunization Schedule 0 5 Yrs Maryland Farms Pediatrics

Covid 19 Vaccines In Pregnancy Myths Vs Facts Virginia Premier

Study Pfizer And Moderna Covid 19 Vaccines Appear Safe For Pregnant People Coronavirus Updates Npr

Faq Answers About Covid 19 Vaccination Alaska Children S Trust
A Fertility Expert Sets The Record Straight On The Covid 19 Vaccine And Fertility The Daily Independent At Yourvalley Net
The Covid 19 Vaccine And Pregnancy Children S National

Information And Resources For Covid 19 And Breastfeeding

Covid 19 Vaccines And Pregnancy Faqs Virtua Health Nj

Pediatric Vaccine Social Media Toolkit
/GettyImages-1237987894-e8c1184324dc458eb541bcb7cd14d7e4.jpg)
Fda Pregnant Women Can Get A Covid 19 Vaccine

Covid 19 International Lactation Consultant Association

Use Shared Decision Making When Offering Covid 19 Vaccine To Pregnant Patients Pharmacy Today

Pregnant Women Should Consider Taking The Covid 19 Vaccine Newsroom

Covid 19 Vaccination During Pregnancy Protects Babies Research Finds University Of Mississippi Medical Center


